Zurich Joint: An Actuated Human Joint Organoid on Chip (ZJ)
Motivation & relevance
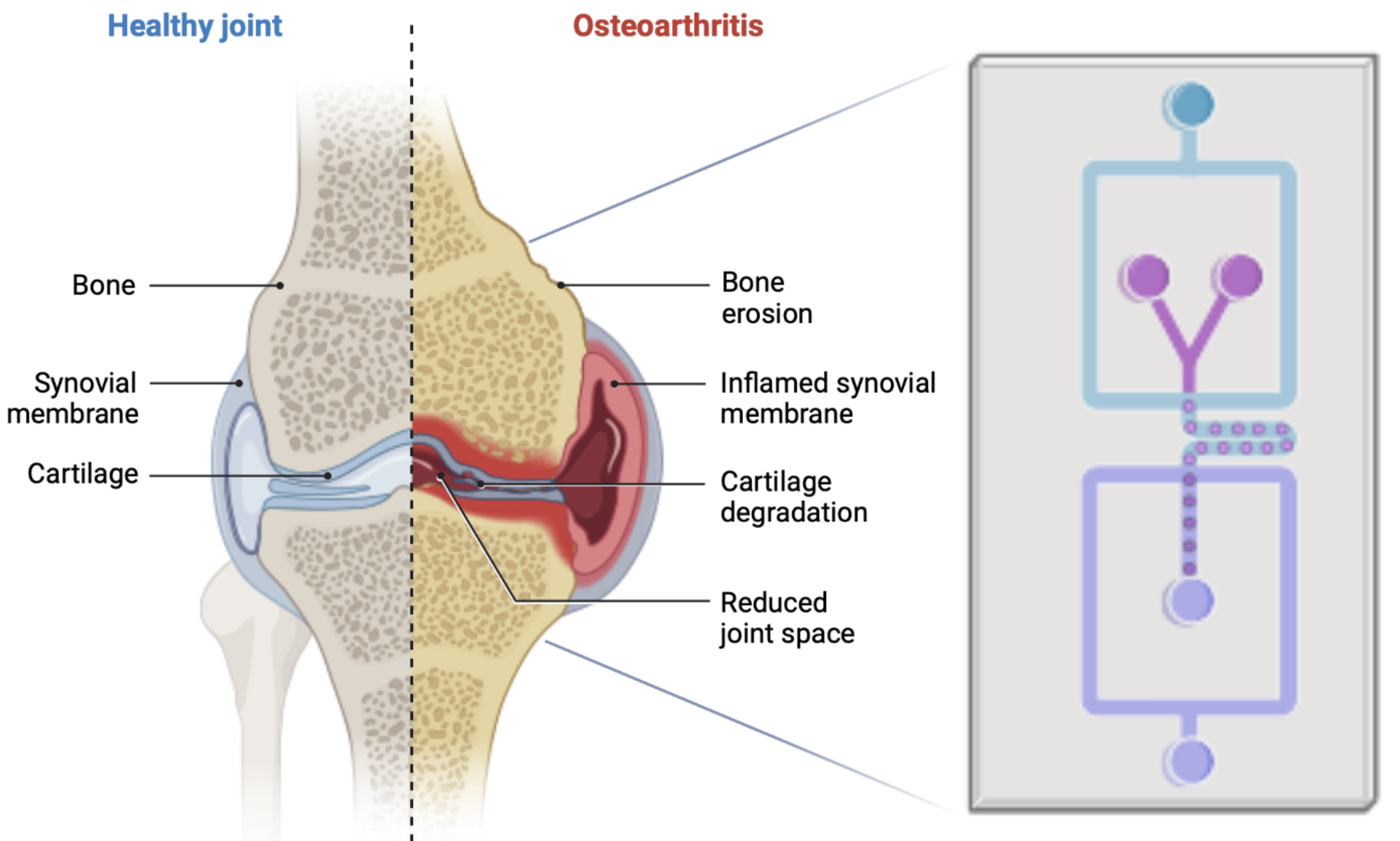
Treating human joint diseases such as osteoarthritis (OA) has been a major societal challenge, especially for the aging population. Traditional in vitro cultures and animal models suffer from limited predictive power for human biology. Developing a microphysiological in vitro biosystem that mimics the complex environment of a human joint holds the potential to revolutionise the methods in medical research and to discover new therapeutics to treat joint diseases.
Key objectives
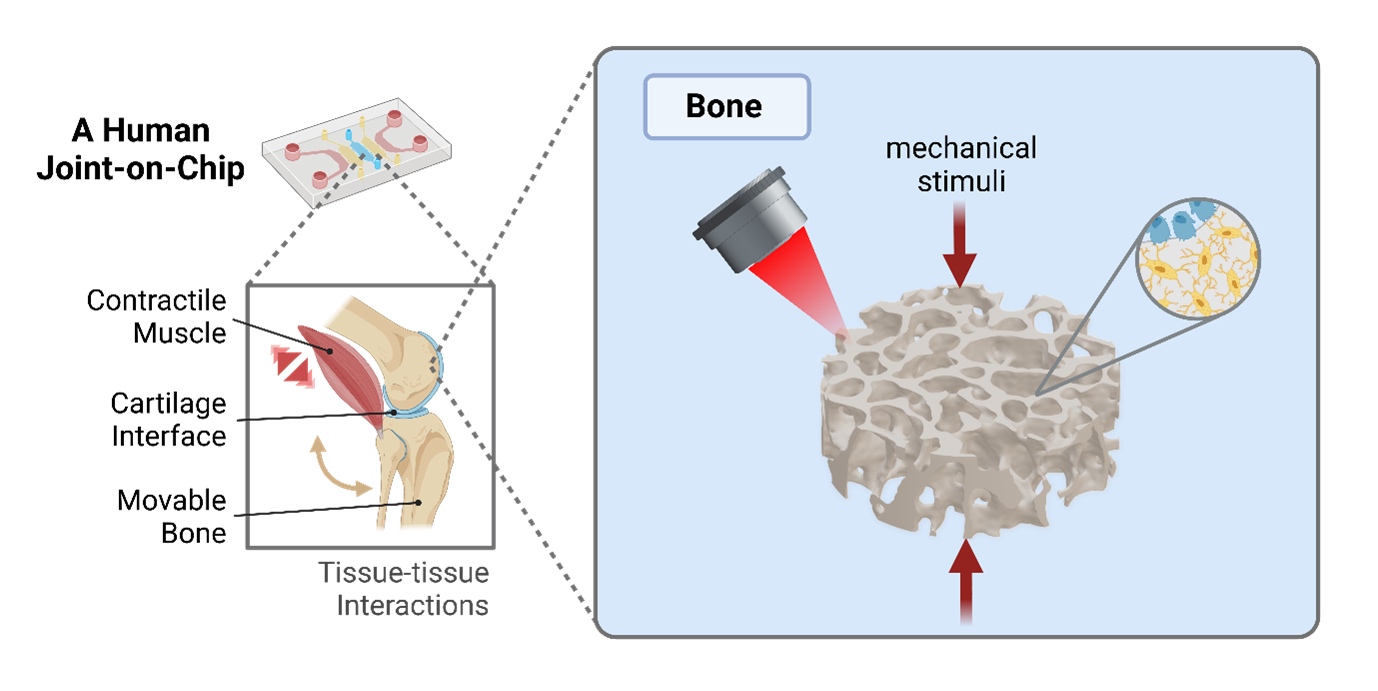
The overall goal of this project is to create an in vitro organoid model of the human joint that comprises functionally connected living tissue components (bone, cartilage, tendon/ligament, muscle) under controlled biomechanical actuation on a chip. The different tissue components of this system will be custom built by 3D bio-printing of patient-derived stem cells and then connected to a multi-organoid-on-chip system in a modular way. The tissue components can be mechanically actuated through optogenetic actuation of integrated muscle cells. The resultant functional adaptation of tissue components can be monitored by multiscale bio-imaging techniques using engineered cells. Using this in vitro platform in combination with computational modelling, we seek to gain a deeper understanding of the role of mechanical stimuli in joint development and growth as well as joint homeostasis and disease, such as how altered mechanical loading leads to joint disease progression. Furthermore, this research has the potential to uncover how different joint components functionally cooperate with each other to allow skeletal movement in order to replace animal models in the spirit of 3R.
Rationale & methods to be employed
To recreate the hierarchical architecture in different joint tissues, we will exploit a multiscale bio-manufacturing approach, including two-photon and volumetric bio-printing with resolutions in the range of 200 nm to 100 μm. We will combine human induced pluripotent stem cells (iPSC) with photo-curable hydrogel bioinks for in vitro 3D tissue differentiation. Moreover, engineered cells (e.g. osteocytes, chondrocytes, tenocytes) will be incorporated into the system to serve as the sensory units for real-time read-outs of joint development by live-cell imaging. Furthermore, we will explore optogenetic modulation of the activity of muscle cells for spatiotemporal control of mechanical actuation in a user-dictated fashion.
As the actuation progresses, cells continue to synthesise tissue-specific extracellular matrices and build tissue-tissue interfaces towards a long-term functional joint organ with a lifetime of months to one year for disease modelling and drug testing applications. Results on the development of muscle tissue will be exchanged with the research efforts on biohybrid robotics. Experiments will be integrated with simulations which, once calibrated and validated by experiments, will guide the growth process by reverse-engineering the required applied multiphysics stimuli. The cellular connectomics in the tissue components under physiological and pathological conditions will be compared in synergy with the microbial systems project.
Impact, expected output & added value of project
This project addresses a major societal challenge through the development of a joint-on-chip in vitro platform. The successful application of this research in the next years may eventually enable researchers to predict how individual patients respond to drug treatments without the use of preclinical animal models. This research is by its nature cross-disciplinary, combining tissue engineering, biomechanics, stem cell engineering, organ-on-chip technology, soft robotics, and computational mechanics. This research will foster medical translation together with clinical partners in Zurich towards a prototype of a medical product. Also, it will strongly advance 3R research (reduction, refinement, and replacement of animal experimentation) at ETH Zurich.
Projects
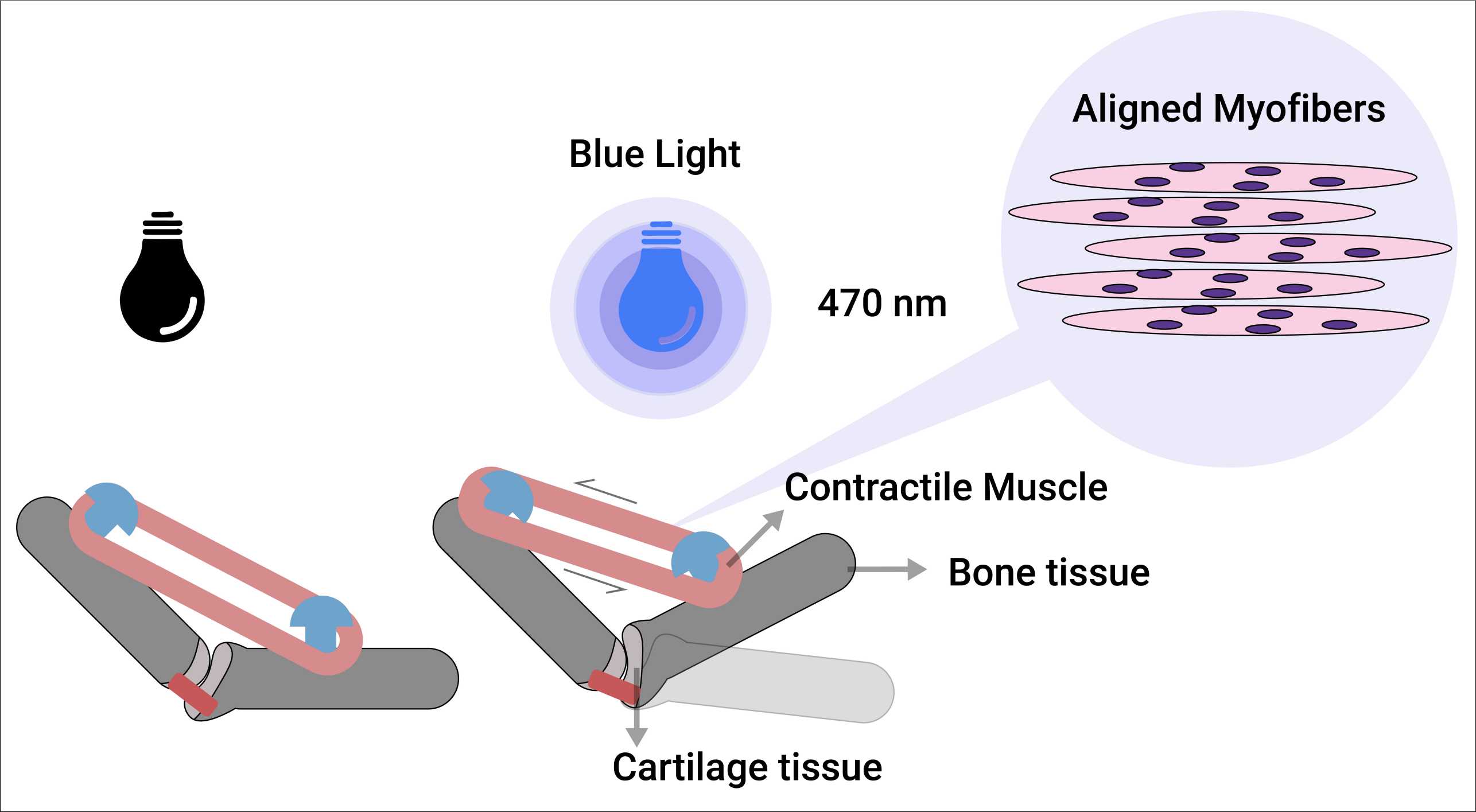
Developing Optically-Driven Contractile 3D Human Skeletal Muscle Bioconstruct for an Actuated Joint-on-chip System
Our objective is to generate a 3D-engineered human muscle bioconstruct that exhibits strong alignment and can contract in response to light stimulus as a part of an actuated joint-on-chip model. This muscle bioconstruct model is expected to allow interrogating the pathophysiology of human muscle, and further serve as a valuable platform for conducting high-throughput drug testing for muscle diseases, notably reducing the need for animal experimentation.
Development of an Actuated Human-Engineered Cartilage-on-Chip Model for Investigating Joint development and Osteoarthritis Pathophysiology
Our study aims to create an actuated cartilage-on-chip model using human-engineered hyaline cartilage that closely resembles the structure and biochemical composition of human cartilage. By employing this model, we aim to investigate the effects of mechanical stimuli and different inflammatory cytokines on cartilage growth and the subsequent development of joint diseases.
Read more
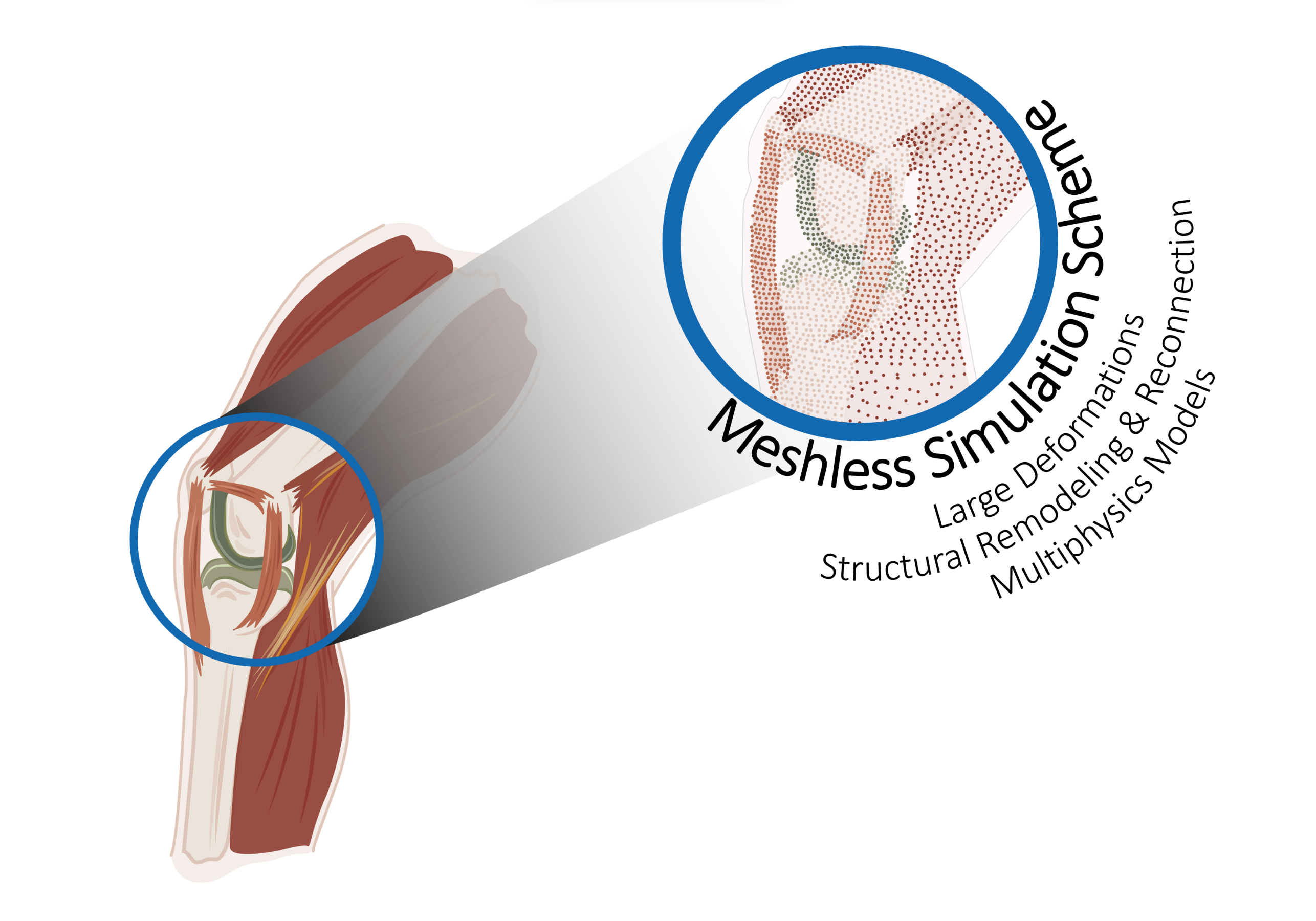
A Computational Framework to Simulate Bio-Multiphysics Problems under Large Deformations
We aim to develop a multiphysics computational model of a human knee joint-on-chip's structural response and growth. To achieve this goal, we will improve on the available bio-chemo-mechanical models to predict the behavior of the constituent tissues and their interactions, and the numerical tools to describe and predict their response.